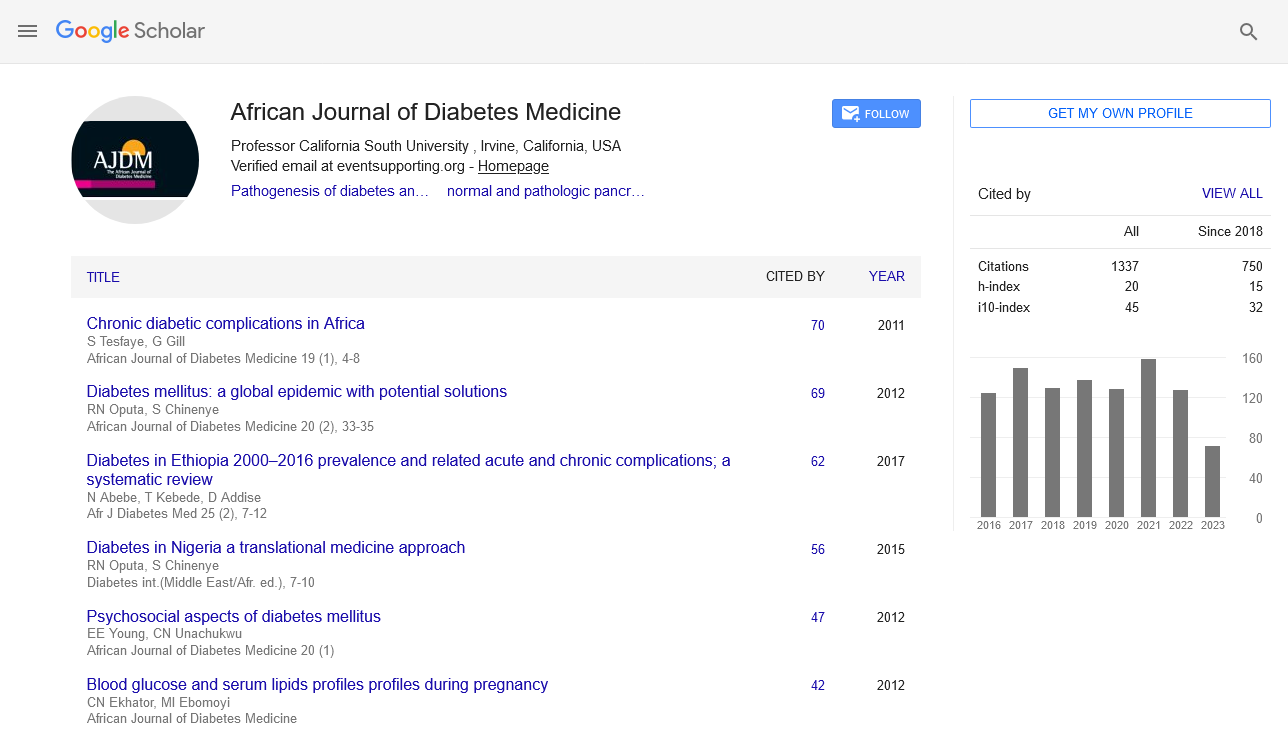
Diabetic retinopathy cascade begins with chronic hyperglycemia affecting the normal progression of glycolysis
*Corresponding Author:
Received: 30-Nov-2022, Manuscript No. ajdm-23-85681; Editor assigned: 02-Dec-2022, Pre QC No. ajdm-23-85681 (PQ); Reviewed: 16-Dec-2022, QC No. ajdm-23-85681; Revised: 21-Dec-2022, Manuscript No. ajdm-23-85681 (R); Published: 28-Dec-2022
Description
Diabetic retinopathy is generally thought to be an inflammatory process. It begins as vascular occlusion and ischemia and can lead to macular edema. The eye adapts through vascular proliferation, which can lead to blindness due to hemorrhage and fibrosis. Complications in diabetic patients are believed to be the pathological consequences of a single underlying cause, hyperglycemia. Patients with type I diabetes show signs of retinopathy within 10-15 years, and those with type II diabetes show signs of non-proliferative diabetic retinopathy after 16 years. There is no doubt that diabetes is a significant individual and public health problem. Uncontrolled hyperglycemia contributes to the development of diabetes complications, ultimately leading to poor health and quality of life. Therapies that target the specific progression of DR seek to limit vision loss and other consequences of pathogenic vascular insufficiency.
Vascular Endothelial Growth Factor (VEGF) is an integral part of the angiogenic process and is involved in retinal neovascularization during PDR. Anti-VEGF therapy has been approved by the US Food and Drug Administration (FDA) for the treatment of age-related macular degeneration, and several anti-VEGF drugs for DME and PDR are currently being tested. In this paper, we examine the mechanisms behind diabetic retinopathy and analyse the potential efficacy and limitations of anti-VEGF drugs for treating diabetic complications.
Impaired pericyte function combined with associated basement membrane thickening and dysfunction ultimately lead to the development of micro aneurysms and punctate intra-retinal hemorrhages. These are two of the earliest abnormalities that an ophthalmoscopic examination can detect. Retinal leaks develop and vasoconstrictors provide a hypoxic environment. Finally, capillaries consist only of tubes of thickened basement membrane and are highly thrombogenic, leading to greater ischemia. Exacerbation of ischemia leads to angiogenesis-mediated transition from non-proliferative diabetic retinopathy to proliferative diabetic retinopathy.
Hypoxia and ischemia characteristic of diabetic retinopathy increase PDGF transcription and contribute to angiogenesis. PDGF is required for normal retinal neovascularization, and its overexpression is a key mediator in the pathogenesis of proliferative diabetic retinopathy. The diabetic retinopathy cascade begins with chronic hyperglycemia that interferes with the normal process of glycolysis. Downstream effects include pericyte injury and/or deregulation of contractility, disruption of micro vascular integrity, and hypoxia-induced imbalance in VEGF signalling. This imbalance leads to uncontrolled development of fragile and highly leaky new blood vessels, predisposing to bleeding and visual obstruction. Therapies that interfere with this inflammatory process must balance the beneficial effects of reduced angiogenesis with the requirement not to damage the epithelia and neurons that support normal vasculature or related functions.
VEGF is a key regulator of angiogenic processes and has been implicated in the development of diabetic retinopathy. Anti-VEGF therapy is designed to reduce retinal neovascularization. Therapies under the anti-VEGF umbrella have been shown to enhance visual acuity in DME and reduce neo-vascular development in PDR compared to standard photocoagulation. Although these treatments are effective, they are not without their drawbacks. In particular, violation of the blood-retinal barrier by anti-VEGF agents can have devastating systemic effects. Furthermore, the long-term effects of these agents are still unknown. VEGF plays such an important role both in the eye and systemically that general inhibition of its action can lead to significant side effects after years of use.
Acknowledgment
None
Conflict of Interest
The author has nothing to disclose and also state no conflict of interest in the submission of this manuscript.